2024 WNAR/IMS/Graybill Annual Meeting
FORT COLLINS, COLORADO
Save the date! The 2024 WNAR/IMS/Graybill meeting will be in Fort Collins, Colorado from Sunday, June 9 through Wednesday, June 12. This year's WNAR/IMS conference will be joint with the 2024 Graybill Conference to be held at Colorado State University.
- The theme of the Graybill Conference is "Rare Disease Drug Development" (the webpage of Graybill Conference can be found here: https://statistics.colostate.edu/graybill-conference-2024/)
- For the WNAR/IMS conference all topics are welcomed.

There will be short courses, a plenary lecture, a Graybill keynote speech and keynote panels from international regulators, invited and contributed sessions, young investigator events, and a Student Paper Award with oral sessions. Prince Allotey (UW) and Catherine Lee (Kaiser Permanente) are the WNAR Program Chairs, Jie Peng (UC Davis) is the IMS Chair, Wen Zhou (Colorado State) is the Local Organizer and Graybill Co-organizer, Jingling Ye (BeiGene) is the Graybill Program chair, and Kayleigh Keller (Colorado State) is the Student Competition Chair. Email wnarprogramchair@gmail.com with questions.
Fort Collins is 65 miles (105 km) north of Denver, approximately 2 hours from major ski resorts and 45 minutes from the Rocky Mountain National Park. The city has a thriving arts scene and an extensive mix of outdoor recreation activities. Visit www.visitftcollins.com to learn about this beautiful area.
To learn more about the Colorado State University campus, visit www.colostate.edu
Important Dates
November 1, 2023: deadline for submission of 2024 WNAR/IBS Outstanding Impact Award and Lectureship
January 10, 2024: deadline for submission of invited session proposals (submit here)
February 15, 2024: deadline for Contributed Oral abstract submissions (submit here)
February 15, 2024: deadline for Student Paper abstracts (submit here)
April 8, 2024: deadline for full paper submissions of students participating in Student paper competition
Registration
The registration site is: https://conferencereg.colostate.edu/WNAR-IMS-Graybill-2024.
- Registration deadline is May 6, 2024
- After registering, you'll get a confirmation email detailing how to book discounted hotel rooms through WNAR. Availability is limited, so register early to secure your spot.
- Limited on-campus housing is available on a first-come, first-served basis.
- Complimentary parking permits and a free shuttle from Denver International Airport to Fort Collins on June 9 and 12 are available in limited quantities. Register soon to reserve yours.
- Upon the approval of organizing committee, cancellation requests must be received by the Office of Conference Services at (970) 491-7501, Fax (970) 491-7747, or e-mail conferences@colostate.edu no later than 11:59pm MST, May 10, 2024. A $60 processing fee will be charged for each cancellation. No cancellation requests will be honored after this time, though substitutions will be allowed.
- Note on Mar 11th, 2024: Due to a platform issue, it occurs that some payment sand registrations have received error messages. We are working with the platform to fix this. Meanwhile, if you encounter this type of error message during registration, please hold out one more day. Also, let us know if you have this issue.
- Update on Mar 17th, 2024: the above issues have been largely resolved:
- If you are paying online with a credit and receive an error message after submitting your payment information, please do not attempt another payment. Please allow one business day for your booking to be confirmed and to receive your confirmation email.
- Update on Mar 31st, 2024: Reminder for registration.
- The discount for HILTON FORT COLLINS has expired on Mar 29th.
- The discount for BEST WESTERN UNIVERSITY INN will expire on May 25th.
- Details about the discounted hotel rooms through WNAR are only available in the confirmation email upon successful registration. Please register at your earliest convenience.
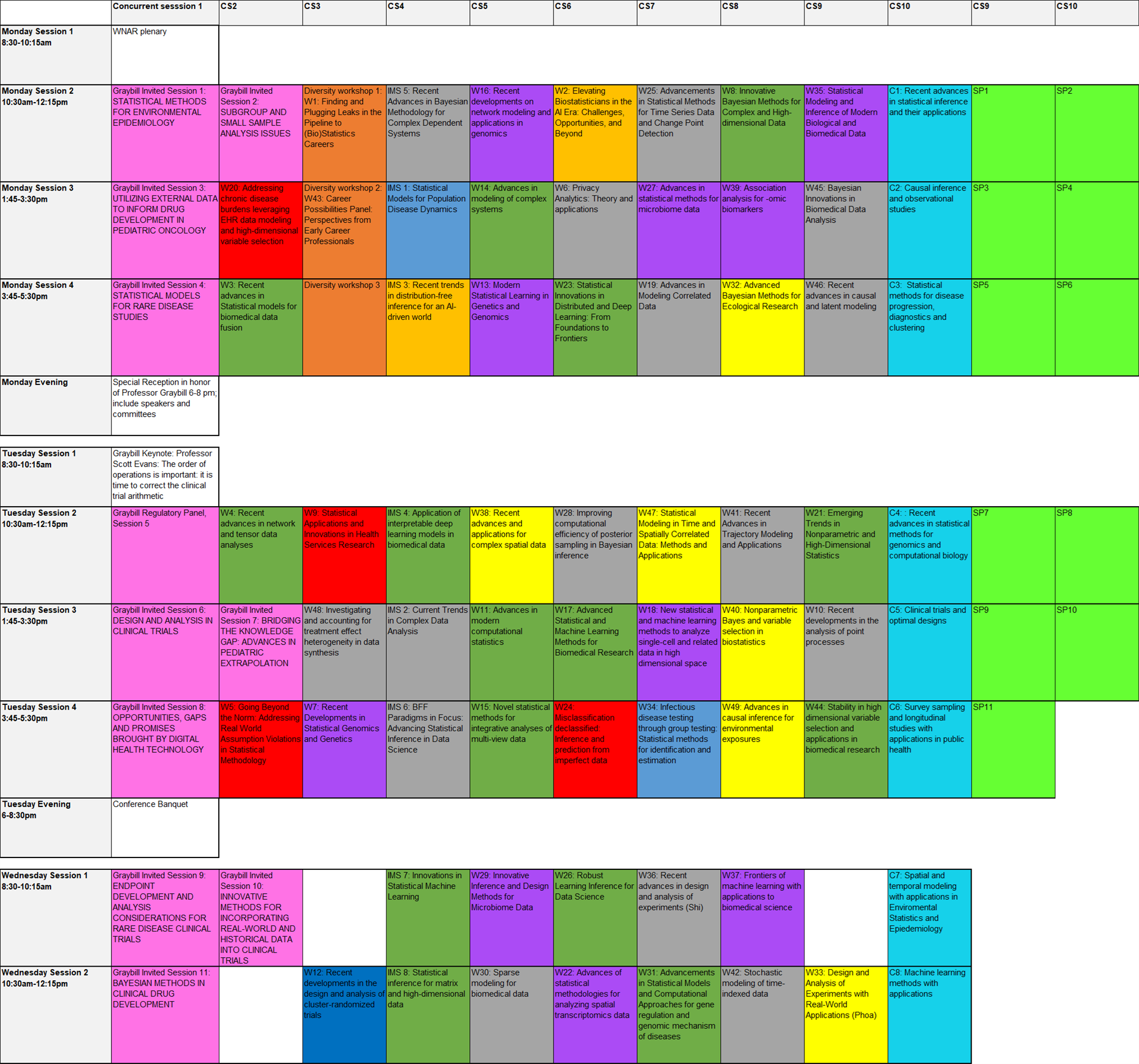
Conference Schedule
WNAR Indigenous Student Travel Awards
WNAR is offering multiple travel awards for Indigenous students from within the WNAR region to attend our annual conference. Awards up to $1,000 are available for conference travel and lodging related expenses. Eligible students include Indigenous peoples of North America and the Pacific Islands. To apply, please send a letter outlining your connection to Indigenous peoples and why you are looking forward to attending the WNAR annual conference to: wnar@wnar.org. Deadline for applications is May 1, 2024.
Accommodations
Hotel accommodations and on-campus housing will be available. More information to come.
Student Paper Competition
WNAR holds a student paper competition each year in conjunction with its annual conference. Awards for Outstanding Written Paper and Outstanding Oral Presentation include a certificate of recognition and a $500 cash prize.
All eligible (students within WNAR region) entrants receive discounts for short course registration and $500 to support travel. For details on eligibility, guidelines for manuscript preparation, and review criteria, please visit the link.
- Students must submit their abstract on or before February 15, 2024.
- More information about the student paper competition can be found here.
- Conference registration is free for all student members of IBS.
- The Student Paper Competition Committee Chair is Kayleigh Keller (wnar2024.student.comp@gmail.com).
- Papers should be submitted to the email address here.
- The deadline for paper submission is Monday April 8, 2024.
- Only one paper submission is allowed per student.
Special Issue
Attendees of the 2024 WNAR/IMS/Graybill Annual Meeting are invited to submit their work for publication in a special issue of theJournal of Data Science. All topics are welcome. Contributions can target any of the JDS sections, including Philosophy of Data Science, Statistical Data Science, Computing in Data Science, Data Science in Action, Data Science Review, Education in Data Science, and Data Science Conversation. Manuscripts should be submitted via the journal's online submission portal https://www.e-publications.org/ruc/sbs/JDS/login. In your cover letter (comments to the editor), please state that your submission is for the WNAR/IMS/Graybill special issue and provide the names and contact information of 3 or more suggested reviewers who would be willing to review your manuscript. All submitted manuscripts must contain original, unpublished work that is not under consideration for publication elsewhere.
Submission inquiries should be directed to the special issue guest editors, Tianjian Zhou (tianjian.zhou@colostate.edu) and Brian Wiens (brian.wiens@acelyrin.com), and Tianying Wang (tianying.wang@colostate.edu). The tentative submission deadline is July 1, 2024, with the possibility of extensions upon contacting the guest editors.
Short Courses
- WNAR will host a short course on Sunday June 9, 2024, 9am-5pm with an hour for lunch.
-
- 1) Adaptive and Bayesian Methods for Clinical Trial Design
-
- Instructor: Alex Kaizer (University of Colorado Anschutz Medical Campus)
- Description: Clinical trials and study designs have evolved rapidly in the past decades as adaptive designs and Bayesian methods have seen become more accessible through software, research, and use. This short course will expand upon both adaptive trial designs, Bayesian methods, and where the two may work synergistically in addition to more traditional frequentist approaches. Attendees will learn through motivating contexts, statistical methods, case studies, and software examples:
- Basics of clinical trial designs
- Interim monitoring for efficacy and/or futility
- Sample size re-estimation approaches
- Adaptive enrichment designs
- Adaptations to treatment arm selection
- Adaptations to randomization ratios
- Seamless trial designs
- Bayesian methods for information sharing/borrowing historic data
- Master protocol designs
- Graybill will host four short courses on Sunday June 9, 2024, 9am-5pm with an hour for lunch.
-
- 1) N-of-1 Trials for Personalized Healthcare
-
- Instructor: Christopher Schmid (Brown University)
- Description: Personalized (N-of-1) trials hold great promise for broadening the clinical knowledge production enterprise to engage individuals in trial design, creation and use of personal data, and decision making. N-of-1 trials use a multi-crossover design in which each individual receives two or more treatments multiple times in a randomized order. In contrast to traditional clinical trial designs, N-of-1 designs can measure individual treatment efficacy to create personalized knowledge. While frequently used to assess treatments for chronic conditions or lifestyle choices, these designs are also uniquely positioned to provide information about the efficacy of treatments for rare diseases and clinical conditions for which recruitment of large numbers of participants is impractical or which may need a personalized treatment protocol. N-of-1 trials may be deployed in a variety of ways. Individuals may create unique, personal designs focused on treatments and outcomes of interest carried out in a manner best suited to them. Or, when practical, trials may be coordinated to have similar protocols facilitating the sharing and combining of information to learn about groups of individuals as well. Such designs may better inform individuals too through borrowing of strength from the findings of exchangeable group members. Such group designs may be particularly valuable in clinical settings such as healthcare organizations that provide personalized care to groups of individuals. By combining individual trials in a multilevel structure, it is also possible to describe average treatment effects in populations and subgroups and measure treatment effect heterogeneity to create generalizable knowledge. We discuss the promise and challenges of N-of-1 trials, including the use of software to design and analyze trials, the use of mobile apps to facilitate participation, retain interest, collect data and provide interpreted results to participants, and some of the research barriers that need to be overcome, particularly the challenges of accommodating personalized protocols. These issues are illustrated by several of our recent projects each involving many N-of-1 trials in which we combined mobile device applications with server-driven statistical analytics using an R package to return results to individuals. We discuss defining treatments and sequences of treatments, synthesizing treatment networks, incorporating patient-specific prior information, automating the choice of appropriate statistical models and assessment of model assumptions, and automating graphical displays and text to facilitate appropriate interpretation by non-technical users.
- 2) The DOOR is Open: A Patient-Centric, Pragmatic Approach to Clinical Trials based on Benefit:risk N-of-1 Trials for Personalized Healthcare
-
- Instructor: Scott Evans and Toshimitsu Hamasaki (George Washington University)
- Description: Randomized clinical trials are the gold standard for evaluating the benefits and harms of interventions but they often fail to provide the necessary evidence to inform medical decision-making (DeMets and Califf, JAMA 2011, 305:713-714). One primary reason for this is a lack of awareness of the nature of benefit:risk, which is the most important question when treating patients in clinical practice. This is the motivation for using a patient-centric, benefit:risk approach to the design, monitoring, analysis, interpretation and reporting of clinical trials and medical product development. Standard approaches to benefit:risk evaluation synthesizing information obtained from separate marginal analysis of each outcome do not address the most important questions for clinical practice as they are not patient-centric. They fail to incorporate associations between outcomes and recognize the cumulative nature of outcomes in individual patients, suffer from competing risk complexities in the interpretation of component outcomes, and since efficacy and safety analyses are often conducted on different populations, generalizability to patient populations is unclear. Treatment effect heterogeneity is typically evaluated based on a single efficacy or safety endpoint, and rarely evaluated based on benefit:risk. These challenges can be addressed by placing increased emphasis on patient-centric benefit:risk evaluation and questions of a pragmatic origin to match their clinical importance. These challenges can be addressed by placing increased emphasis on patient-centric benefit:risk evaluation and questions of a pragmatic origin to match their clinical importance. In this short course, we will discuss the patient-centered method of assessing benefit:risk, called the Desirability of Outcome Ranking (DOOR). The desirability of outcome ranking (DOOR) is a paradigm for the design, analysis, interpretation and reporting of clinical trials and other research studies based on patient-centric benefit:risk evaluation (Evans SR, Rubin D, Follmann D et al. Clin Infect Dis. 2015; 61:800-806, Evans SR, Follmann D. Stat Biopharm Res. 2016;8:386-393). The DOOR methodology uses outcomes to analyze patients rather than patients to analyze outcomes by comparing the experiences of trial participants in different treatment arms by the desirability of the overall patient outcome. The motivation for DOOR is to address the above limitations, increasing pragmatism and addressing the most important “real world” question to aid clinical decision-making: how do resulting patient experiences, when comprehensively considering benefits and harms, compare between therapeutic alternatives? In this course, we present several ways to define a DOOR outcome, which represents a global patient response constructed on the basis of important clinical outcomes. We describe two complementary statistical approaches for analyzing clinical trial data using the DOOR methodology, with applications to clinical trials in infectious diseases and other disease areas. We present an interactive web-based tool for implementing the DOOR methodology that allows statisticians and clinical researchers to easily perform the analyses. We discuss methods for sizing clinical trials with the DOOR outcome as the primary endpoint. Finally, we briefly discuss further developments in design and analysis of randomized trials using the DOOR methodology, including (1) subgroup analyses, (2) benefit:risk evaluation in longitudinal and survival trials, (3) integrated benefit:risk analysis of multiple trials, and (4) group-sequential and adaptive designs for monitoring trials.
- 3) Small Sample, Sequential, Multiple Assignment, Randomized Trial (snSMART) Designs and Methods for Chronic, Rare Disease Drug Development
-
- Instructor: Kelley Kidwell (University of Michigan)
- Description: Sequential, multiple assignment, randomized trial (SMART) designs are often motivated to identify tailored sequences of treatments or dynamic treatment regimens (DTRs) in larger samples. SMARTs employ at least two randomizations in sequence where only some groups may be re-randomized based on response or other characteristics related to previous treatment. We have turned standard SMART designs and analyses on their head, and instead of focusing on DTRs, we apply the design to small samples to obtain more information from a small sample of individuals. This short course will provide an overview of small sample SMART (snSMART) designs with corresponding Bayesian and frequentist methods for analyses. The differences between snSMART and SMART designs will be highlighted and methods to analyze snSMART data, calculate sample size, add adaptive components, incorporate external data, and dose-find and confirm will be presented. Many of our methods are motivated by the current snSMART ARAMIS which seeks to find an effective treatment for individuals with isolated skin vasculitis, but the methods apply broadly to chronic, rare diseases that remain relatively stable over the trial period.
- 4) Bayesian Borrowing Techniques for Rare Disease Clinical Research
-
- Instructor: Joseph Koopmeiners and Steffen Ventz (University of Minnesota)
- Description: Randomized clinical trials (RCTs) are the gold standard for estimating the effect of a treatment on an outcome. However, RCTs are also resource-intensive and require large samples to estimate moderate or small effect sizes. The resource-intensive nature of RCTs poses particular challenges in the setting of rare diseases where limitations on the number of potential trial participants limit the overall sample size of RCTs. Given these limitations, the design of RCTs in the context of rare diseases places a premium on efficiency and leveraging all available information to evaluate the effect of a treatment on an outcome. One approach to improve the efficiency of RCTs is to leverage external information, in the form of supplemental trial data or real-world data sources (EHR, etc.), through dynamic borrowing. Recent advances in Bayesian methods for dynamic borrowing provide a powerful set of statistical tools to improve the efficiency of RCTs by leveraging data external to the trial. This short course will provide an introduction to Bayesian methods for dynamic borrowing in the setting of rare disease clinical research. Specific topics to be covered include the motivation for leveraging external data in RCTs, an overview of Bayesian methods for dynamic borrowing, including recent advancements in the use of real-world data to augment RCTs, computational tools for implementing these methods, and a general discussion of the strengths and weaknesses of implementing these methods in practice. Throughout the course, methods will be illustrated via case studies from rare disease clinical research.
